electron configuration of sc|A step : Tagatay Click on above elements (in Periodic table) to see their information or Visit . 05-07-2016; You can find the Powerball numbers for Saturday, May 7, 2016 right here. You can see the numbers in drawn order or ascending order, alongside information about the jackpot and the number of winners, plus the Double Play result. The prize table underneath shows all the payouts for each prize level, whether you added Power Play or .
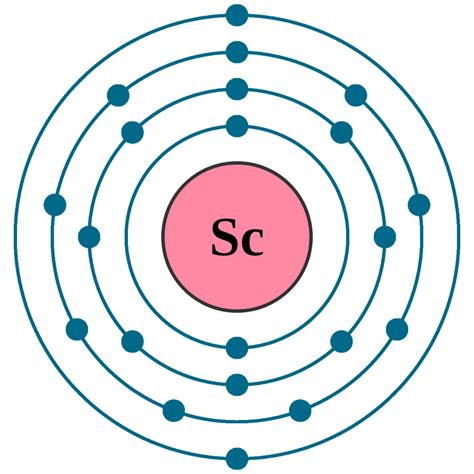
electron configuration of sc,119 rows — Mar 23, 2023 — Electron configuration chart of all Elements is mentioned in the table below. The Shorthand electron configuration (or Noble gas configuration) as well as Full electron configuration is also mentioned in the table. Free Gift for you: Interactive Periodic Table. Let .Click on above elements (in Periodic table) to see their information or Visit .Peb 1, 2021 — Element configuration for atoms or molecules is defined by the number of electrons present in the orbit or shell. In case of Scandium, there are 3 orbits and 17 electrons present in these orbits or shells. Distribution of .
Electron configuration The arrangements of electrons above the last (closed shell) noble gas. Melting point The temperature at which the solid–liquid phase change occurs. Boiling point .A stepKey Takeaways: Scandium’s electron configuration is 1s² 2s² 2p6 3s² 3p6 3d¹ 4s². The electron filling order follows the sequence of 1s, 2s, 2p, 3s, 3p, 3d, and 4s orbitals. Scandium has two .Okt 3, 2020 — A step-by-step description of how to write the electron configuration for Scandium (Sc). In order to write the Sc electron configuration we first need to kn.Scandium electron configuration. ← Electronic configurations of elements. Sc (Scandium) is an element with position number 21 in the periodic table. Located in the IV period. Melting .Nob 13, 2020 — The chemical symbol for Scandium is Sc. Electron Configuration and Oxidation States of Scandium. Electron configuration of Scandium is [Ar] 3d1 4s2. Possible oxidation .
Scandium is a silvery-white transition metal with atomic number 21 and symbol Sc. Its electron configuration is [Ar] 3d 1 4s 2, with valence electrons of 3. Learn more about its history, .By “building up” from hydrogen, this table can be used to determine the electron configuration for any atom on the periodic table. We will now construct the ground-state electron configuration and orbital diagram for a selection of .Nob 4, 2014 — Khanmigo is now free for all US educators! Plan lessons, develop exit tickets, and so much more with our AI teaching assistant.Ene 30, 2023 — Making Sc 2 + Now you are going to add the next electron to make Sc 2 +. Where will the electron go? The 3d orbitals at scandium have a lower energy than the 4s, and so the next electron will go into a 3d orbital. The structure is [Ar] 3d 1. Making Sc + You might expect the next electron to go into a lower energy 3d orbital as well, to give [Ar .Electronic configuration: The arrangement of electrons into the orbitals of an atom using some fundamental principle is called its electronic configuration. Electronic configuration of Scandium: The atomic symbol of Scandium is Sc. The atomic number of Scandium is 21. The atomic number of an element is equal to the number of electrons present .Scandium atoms have 21 electrons and the shell structure is 2.8.9.2. The ground state electronic configuration of neutral scandium is . The mineral thortveitite contains 35-40% Sc 2 O 3 is used to produce scandium metal but another important source is as a byproduct from uranium ore processing, .Electron atomic and molecular orbitals A Bohr diagram of lithium. In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. [1] For example, the electron configuration of the neon atom is 1s 2 2s 2 2p 6, meaning that the 1s, 2s, and 2p subshells .Abr 22, 2024 — Scandium is a chemical element with the symbol Sc and atomic number 21. It is a silvery-white, rare earth metal that belongs to the transition metals group in the periodic table. . Electron configuration. The electron configuration of an element describes the arrangement of electrons in the atoms of that element, and be used to predict its .
Ene 13, 2023 — The electron configurations and orbital diagrams of these four elements are: Figure \(\PageIndex{5}\): Since the core electron shells correspond to noble gas electron configurations, we can abbreviate electron configurations by writing the noble gas that matches the core electron configuration, along with the valence electrons in a condensed .The electron configurations of atoms follow a standard notation in which all the atomic subshells that contain electrons (with the number of electrons they hold written in superscript) are placed in a certain sequence. For example, the atomic number of Sodium is 11 and its electron configuration is 1 s 2 2 s 2 2 p 6 3 s 1. Aufbau principle:-

The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons) are analogous in the electron configurations of their outer shells to their corresponding family members carbon, nitrogen, oxygen, fluorine, and neon, respectively, except that the principal .
electron configuration of sc A stepThe electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons) are analogous in the electron configurations of their outer shells to their corresponding family members carbon, nitrogen, oxygen, fluorine, and neon, respectively, except that the principal .
Hun 14, 2015 — The electron configuration states where electrons are likely to be in an atom. If you don’t have a chart, you can still find the electron configuration. Use the element blocks of the periodic table to find the highest electron orbital. Alternatively, remember group 1 (alkali metals) and group 2 (alkaline earth metals) are s-block, groups 2 .

Scandium is a chemical element of the periodic table with chemical symbol Sc and atomic number 21 with an atomic weight of 44.9559 u and is classed as a transition metal. . Electron configuration chart. 1s 2: 2s 2: 2p 6: 3s 2: 3p 6: 3d 1: 4s 2: Electrons per shell: 2, 8, 9, 2: Valence electrons : 3: Valency electrons : 3:Therefore, the electronic configuration of sulfur can be written as 1s 2 2s 2 2p 6 3s 2 3p 4. The electronic configuration of elements can also be written with the help of noble gases. These noble gases have completely filled outermost .The first three quantum numbers of an electron are n=1, l=0, m l =0. Only two electrons can correspond to these, which would be either m s = -1/2 or m s = +1/2. As we already know from our studies of quantum numbers and electron .Hun 20, 2023 — The third major category of elements arises when the distinguishing electron occupies an f subshell. The first example occurs in the case of the lanthanoids (elements having atomic numbers between 57 and 71).The lanthanoids have the general electron configuration [Kr]4d 10 4f i 5s 2 5p 6 5d 0 or 1 6s 2. where i is a number between 0 and 14. Thus in the .
Hun 27, 2024 — This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Typically, you need at least 8 steps to determine the electron configuration, starting with finding the atomic number by looking at the list of orbitals and understanding the notation.. But wait — you can avoid all this if you use our .B) Explain why the electron configuration of Sc is not [Ar] 3d 3. C) Sc and Ti + are isoelectronic. Explain why their electron configurations are different even though they have the same number of valence electrons. Answer A. Sc: [Ar] 3d 1 4s 2. Sc +: [Ar] 3d 2 *when a d-block ion forms, it pulls the 3d orbital much lower than the 4s so that .The electron configurations and orbital diagrams of these four elements are: The alkali metal sodium (atomic number 11) has one more electron than the neon atom. . Note that for three series of elements, scandium (Sc) through copper (Cu), yttrium (Y) through silver (Ag), and lutetium (Lu) through gold (Au), .
electron configuration of sc|A step
PH0 · Scandium Electron Configuration (Sc) with Orbital Diagram
PH1 · Scandium Electron Configuration (Sc) with Orbital
PH2 · Scandium (Sc)
PH3 · Scandium
PH4 · Electron configuration for Scandium (element 21). Orbital diagram
PH5 · Electron Configuration for Scandium (Sc, Sc3+ ion)
PH6 · Electron Configuration for Scandium (Sc, Sc3+ ion)
PH7 · Electron Configuration For Scandium
PH8 · Electron Configuration Chart of All Elements (Full Chart)
PH9 · A step
PH10 · 3.1: Electron Configurations